 |
 |
|
 |
|
 |
|  |
|  |
|
 |
|
 |
|  |
|  |
|
 |
Pretentious but the result was good.
Thanks to all who helped with the media!
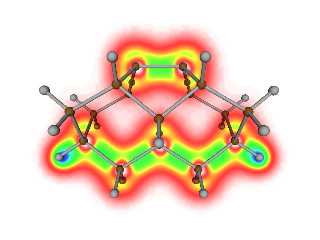
The object is a slice through the total electron density of the surface and
immediate substructure of the silicon <100> crystal plane.
Jim
Post a reply to this message
Attachments:
Download 'semiconductor_splendor.jpg' (36 KB)
Preview of image 'semiconductor_splendor.jpg'
|
 |
|  |
|  |
|
 |
|
 |
|  |
|  |
|
 |
Don't know if I interprete the image right at all but it looks like there's
a large imbalance of electrons between the atoms and yet perhaps it almost
equals out overall (if the entire thing seen).
In other words, the brown atoms show little to no electrons and the light
gray ones have a high density of them. A possibly mostly positive charge
then with electrons ready to jump around?
It's what I see anyway. Just to make an untrained observation.
Bob H.
Post a reply to this message
|
 |
|  |
|  |
|
 |
|
 |
|  |
|  |
|
 |
This image depicts a 2D slice of the electron density contained within a 3D
view of the crystal lattice. So the atoms if particular interest are the
ones that are present in the plane of the slice. The others are there just
to provide a crystal lattice frame of reference.
The density color map is red, orange, yellow, green, blue, indigo, violet
where the lowest electron density regions are red, the highest are violet.
White is the region of no density. Electron density is concentrated around
atoms and between atoms that are chemically bonded to each other. Thus the
yellow/green areas are bonded electron density, the blue are regions of
atomic density.
The brown atoms are silicon. The reason they don't have blue/indigo/violet
color near them (i.e. the core density) is because the particular Quantum
Mechanical method I used to calculate the electron density approximates the
core density and thus it does not explicitly show up on these electron
density images. The gray atoms are hydrogen atoms which do not use that
approximation and thus their core density appears here.
Jim
"Bob H." <omn### [at] msn com> wrote in message
news:3b361bca@news.povray.org...
> Don't know if I interprete the image right at all but it looks like
there's
> a large imbalance of electrons between the atoms and yet perhaps it almost
> equals out overall (if the entire thing seen).
> In other words, the brown atoms show little to no electrons and the light
> gray ones have a high density of them. A possibly mostly positive charge
> then with electrons ready to jump around?
> It's what I see anyway. Just to make an untrained observation.
>
> Bob H.
>
> com> wrote in message
news:3b361bca@news.povray.org...
> Don't know if I interprete the image right at all but it looks like
there's
> a large imbalance of electrons between the atoms and yet perhaps it almost
> equals out overall (if the entire thing seen).
> In other words, the brown atoms show little to no electrons and the light
> gray ones have a high density of them. A possibly mostly positive charge
> then with electrons ready to jump around?
> It's what I see anyway. Just to make an untrained observation.
>
> Bob H.
>
>
Post a reply to this message
|
 |
|  |
|  |
|
 |
|
 |
|  |
|  |
|
 |
"Bob H." wrote:
>
> Don't know if I interprete the image right at all but it looks like there's
> a large imbalance of electrons between the atoms and yet perhaps it almost
> equals out overall (if the entire thing seen).
> In other words, the brown atoms show little to no electrons and the light
> gray ones have a high density of them. A possibly mostly positive charge
> then with electrons ready to jump around?
> It's what I see anyway. Just to make an untrained observation.
This shows that the substance is Polar. Which means there is some place where
you could bind atoms to form a new molecule. A good example of Polar molecule
is water (H2O, the electrons are pulled by the 2 Hydrogen atoms and bindings can
then be easily be done with the uncovered Oxygen atom. A non-polar molecule
would be something like Benzene C6H6 A ring of 6 Carbon atom all of them binded
to one Hydrogen atom, there is no point where the electron density is higher or
lower so (at least in french) we say it has no Poles, and thus it is a non-polar
molecule.
But this silicon molecule shows a very intruiging kind of polarity!
--
|| 'How do you know I'm mad?' said Alice.
|| 'You must be,' said the Cat, 'or you wouldn't have come here.'
--
Simon Lemieux (lem### [at] yahoo com) com)
Post a reply to this message
|
 |
|  |
|  |
|
 |
|
 |
|  |
|  |
|
 |
"Jim Kress" <dea### [at] kressworks com> wrote in message
news:3b3633de$1@news.povray.org...
> This image depicts a 2D slice of the electron density contained within a
3D
> view of the crystal lattice. So the atoms if particular interest are the
> ones that are present in the plane of the slice. The others are there
just
> to provide a crystal lattice frame of reference.
I see, at least I sort of knew that much.
> The density color map is red, orange, yellow, green, blue, indigo, violet
> where the lowest electron density regions are red, the highest are violet.
> White is the region of no density. Electron density is concentrated
around
> atoms and between atoms that are chemically bonded to each other. Thus
the
> yellow/green areas are bonded electron density, the blue are regions of
> atomic density.
Figured on that as well.
> The brown atoms are silicon. The reason they don't have
blue/indigo/violet
> color near them (i.e. the core density) is because the particular Quantum
> Mechanical method I used to calculate the electron density approximates
the
> core density and thus it does not explicitly show up on these electron
> density images. The gray atoms are hydrogen atoms which do not use that
> approximation and thus their core density appears here.
Thanks, explanation enough for me even if I don't understand it 100%. :-)
Bob H. com> wrote in message
news:3b3633de$1@news.povray.org...
> This image depicts a 2D slice of the electron density contained within a
3D
> view of the crystal lattice. So the atoms if particular interest are the
> ones that are present in the plane of the slice. The others are there
just
> to provide a crystal lattice frame of reference.
I see, at least I sort of knew that much.
> The density color map is red, orange, yellow, green, blue, indigo, violet
> where the lowest electron density regions are red, the highest are violet.
> White is the region of no density. Electron density is concentrated
around
> atoms and between atoms that are chemically bonded to each other. Thus
the
> yellow/green areas are bonded electron density, the blue are regions of
> atomic density.
Figured on that as well.
> The brown atoms are silicon. The reason they don't have
blue/indigo/violet
> color near them (i.e. the core density) is because the particular Quantum
> Mechanical method I used to calculate the electron density approximates
the
> core density and thus it does not explicitly show up on these electron
> density images. The gray atoms are hydrogen atoms which do not use that
> approximation and thus their core density appears here.
Thanks, explanation enough for me even if I don't understand it 100%. :-)
Bob H.
Post a reply to this message
|
 |
|  |
|  |
|
 |
|
 |
|  |
|  |
|
 |
Simon Lemieux wrote:
>
> A non-polar molecule
> would be something like Benzene C6H6 A ring of 6 Carbon atom all of them binded
> to one Hydrogen atom, there is no point where the electron density is higher or
> lower so (at least in french) we say it has no Poles, and thus it is a non-polar
> molecule.
But wouldn't the atoms still have slightly different fields than in a
state by themselves because of the difference in electronegativity?
There isn't really a line between non-polar, polar and ionic, chemists
just say that delta-electronegativity from 0.0 to some number (0.7
IIRC?) is a "non-polar" bond, etc. Unless I misunderstand what you're
saying. Do you mean in the sense that benzene is non-polar by its
geometry as well?
--
David Fontaine <dav### [at] faricy net> ICQ 55354965
My raytracing gallery: http://davidf.faricy.net/ net> ICQ 55354965
My raytracing gallery: http://davidf.faricy.net/
Post a reply to this message
|
 |
|  |
|  |
|
 |
|
 |
|  |




![]()