|
 |
Hello,
the attached scene-files produce pictures of the
quantum wave functions of hydrogen.
I've put an example too.
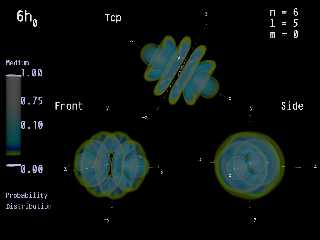
There are two possibilities for viewing. Both are
the density of the media inside a sphere and the
density is color-represented.
Probability Distribution.
This is the chance to find an electron somewhere
around the nucleus. The density is equal to the chance.
Wave Function.
This is the mathematical description of the wave
and the density is equal to the amplitude of the wave.
Version 3_8 is made with hand-derived functions
and is limited to n = 4 (main quantum number).
Version 4_1 is extended to n = 10 and uses
computer generated derivatives. There is a slight
difference between the results, but I can't find the cause
of this. Somehow the waves are a little bit bigger with 4_1.
If you know something about quantum numbers, then
you can go ahead with them directly:
Change the settings (explanation provided) and run it.
Test the settings first with small pictures, for the media
are not so fast.
For those who this stuff is new:
I will keep this as simple as is possible.
In modern science the electron is described as a wave
that has an electrical charge and a mass.
As a result of this, the electron can't move freely around
the nucleus as a rocket around the earth, but has to
comply to certain mathematical rules.
Some (awful) mathematical manipulations result in a
very complicated function that is called the wave-function.
In this function you find three variables that are natural
numbers( 0, 1, 2, ...) and these are called the quantum
numbers:
('l' : this is the letter 'l' that follows the letter 'k'. With my font
this is almost equal to the number 1. I'll put them between
''.)
'n' = main quantum number ('n' = 1, 2, 3, ..)
This influences the radius of the wave. In short: how big.
'l' = second quantum number (officially the azimuthal
quantum number) ('l' = 0, 1, .., 'n'-1)
This influences the model of the wave and is equal
to the number of knots in the wave.
You can vary 'l' between 0 and 'n'-1.
'm' = third quantum number (officially the magnetic
quantum number) ('m' = -'l', .., 0, .., +'l')
This influences directions in space.Moving
electrical charges generate magnetic fields
and this number has something to do with that.
You can vary 'm' between -'l' and +'l'.
Let us give an example:
You choose 'n' = 3.
You now can vary 'l' : 0, 1 and 2 . Choose 'l' = 1.
You now can vary 'm': -1, 0, 1
Choose the combination 'n = 3, 'l' = 1 and 'm' = 0
and you get the quantum wave that is called 3p0.
There are more things that you can choose, but
I hope that the explanation is sufficient. You
can always ask!
Regards,
Jaap Frank
Post a reply to this message
Attachments:
Download 'H_Wave_Functions v3_8.pov.txt' (45 KB)
Download 'H_Wave_Functions v4_1.pov.txt' (31 KB)
Download 'H_PROB_6H0_MED.png' (184 KB)
Preview of image 'H_PROB_6H0_MED.png'
|
 |




![]()